Dinodornox Liquid®
FOR THE EFFECTIVE BIO-CONTROL OF SLUDGE AND AMMONIA EMISSIONS
100 % NATURAL, WILL NOT HARM LIVESTOCK OR HUMAN BEINGS
Controls biological sludge and odor associated with livestock production
WHAT IT DOES
♦ DINODORNOX LIQUIDT M Metabolizes the ammonia present in the waste on contact, eliminating the odors from the pens, channels and ponds.
♦ DINATEC has created DINODORNOX LIQUIDTM to solve these roblems. Improve bio‑filter performance.
♦ DINODORNOX LIQUID TM is environmentally friendly and since it eliminates ammonia and liquefies sludge, accidental releases from collection ponds will not harm surrounding surface water. Liquid from the collection ponds can be sprayed on soils, enriching them similar to an application of compost.
DINODORNOX LIQUID is more effective and environmentally friendly.
To improve the effectiveness of our biological treatment, steps are taken to enhance and improve with DINODORNOX LIQUID, the performance of various microbial constituents in our formula. Bacteria are isolated from soils and water,
then elevated in the laboratory to identify respective abilities to degrade specific chemical structures. The cultures are tested for their response to environmental variables and further analyzed to select the genetically superior examples of each desirable strain. Once differentiated, the microbes reproduce, and are combined into formulations which will further maximize the efficiency of the
biological treatment. Our products are typically made of a number of species which have been specifically cultured. These organisms work together, each strain possessing the enzyme system necessary to degrade certain components of a targeted waste, yielding intermediate breakdown products that can be further degraded by other species formulated in the blend. These organisms can also out compete pathogenic bacteria.
GMP
DINODORNOX LIQUID is produced by procedures that adhere to Good anufacturing Practices, (GMP). All formulations are made by following strict Standard Operating Procedures (SOP), including complete Quality Control
(QC) testing of raw ingredients, the finished product, and sample retention.
DINODORNOX LIQUIDTM May be applied to lagoons used to raise fish and shrimp. It will reduce the levels of ammonia and nitrite in the water as well as the organic sludge build-up resulting from over-feeding and fish and shrimp waste. Regular applications of DINODORNOX LIQUIDTM during the growing cycle will allow the lagoon to support larger, more robust and healthier populations of fish and shrimp.
WATER QUALITY PARAMETERS
Start at 20% water turnover rate and monitor. Maintain oxygen levels at 5 ppm minimum. Effective pond pH range is 5.5-7.5.
DIRECTIONS FOR USE DINODORNOX LIQUIDTM. STIR WELL BEFORE USING
Ammonia (NH4) Emissions Test Results
Starting 10-days after hatching 3 houses were treated with DINOdornox Liquid, 2 were not. Ammonia, (NH4) masurements were taken at 20, 35-days and in the empty houses.
DIRECTIONS FOR USE
DINODORNOX LIQUIDTM
STIR WELL BEFORE USING
Ammonia (NH4) Emissions Test Results
Starting 10-days after hatching 3 houses were treated with DINOdornox Liquid, 2 were not. Ammonia, (NH4) masurements were taken at 20, 35-days and in the empty houses.
DINODORNOX LIQUID is a 100 % natural biological Control of Sludge and dors associated With Livestock Waste Treatment
| Interval | Houses W/O DINOdornox (ppm) | With Di-nodornox (ppm) | Reduction (%) |
|---|---|---|---|
| 20-Days | 5.00 | 2.00 | 60.00 |
| 35-Days | 30.00 | 10.00 | 66.00 |
| Empty | 30.00 | 17.00 | 43.00 |
| Average | 21.67 | 9.67 |
56.33 |
| DINODORNOX LIQUID™ | Quantity of Water | ||
|---|---|---|---|
| Weekly | Every 3 Days | Weekly | Every 3 days |
| 10 ml | 5 ml | 100 ml | 100 ml |
| 100 ml | 50 ml | 1000 ml | 1000 ml |
| 1000 ml | 500 ml | 10,000 ml | 10,000 ml |
| DINODORNOX | Area Covered |
|---|---|
| 100 ml | 20 m2 |
| 1000 ml | 200 m2 |
| 10,000 ml | 2000 m2 |
To Treat Poultry Hog & Livestock Enclosures Apply as Follows:
Initial Treatment
Dilute 1 liter of DINODORNOX LIQUID ™ with at least 500 liters of de-chlorinated water. Spray on floors, poultry litter and walls to cover an area of 1000m2. Apply twice weekly for the first two weeks.
Maintenance Treatment
Dilute 1 liter of DINODORNOX LIQUID™ with at least 500 liters of water. Spray on floors & walls to cover an area of 2000m2. Apply twice per week.
Caged Layer Farms
Dilute DINODORNOX LIQUID™ with water and spray on cage bottoms every 3 days or once a week using the following dilution table.
USE IN LAGOONS
Initial Treatment
Normal ponds use a 1:30,000 dilution ratio. (33.3 PPM) Heavily contaminated ponds use a 1:20,000 ratio (50 PPM) Stir prior to use. Initial treatment, spray evenly over the entire water surface. Maintenance treatments can be poured into the inflow line to the lagoon or metered in using a pump. Optimum treatment conditions are water pH of 6 to 9, water temperature above 50o F, and a Total Dissolved Oxygen concentration greater than 4 ppm. Aeration of the lagoon will increase the effectiveness of the treatment but is not mandatory.
AVAILABLE PACKAGING
20 liter Plastic Pails or
210 liter Plastic Drum
STORAGE DIRECTIONS
Store in a cool dry environment. Do not allow to freeze. Keep away from direct exposure to sunlight.
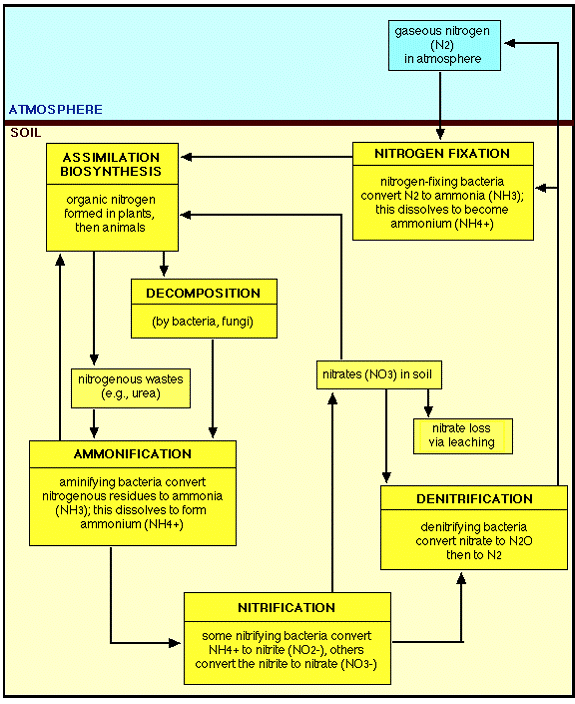
THE NITROGEN CYCLE
The nitrogen cycle is composed of four processes. Three of the processes–fixation, ammonification, and nitrification–convert gaseous nitrogen into usable chemical forms. The fourth process, denitrification, converts fixed nitrogen back to the unusable gaseous nitrogen state (Smith, 1990).
Nitrosomonas is the first bacteria the Nitrogen Cycle produces to remove the organic waste product ammonia from concentrating in an aquarium. nitrosomonas is a lithotrophic bacteria that requires clean, hard surfaces to atttach its population to. It is aerobic, requiring an adequate supply of oxygen to sustain its life cycle as well. Most often, these bacteria are cultured in undergravel filters which provide the clean hard surfaces they need to attach to. The nutrients provided by the water as it flows through the gravel substrate and under the undergravel plate are normally suitable to produce enough of a population to remove all the ammonia from the aquarium as soon as it is produced by the fish and decay processes.
Nitrogen fixation is the conversion of nitrogen in its gaseous state to ammonia or nitrate. Nitrate is the product of high-energy fixation by lightning, cosmic radiation, and meteorite trails. In high-energy fixation, atmospheric nitrogen and oxygen combine to form nitrates, which are carried to the earth’s surface in rainfall as nitric acid. High-energy fixation accounts for little (10%) of the nitrate entering the nitrogen cycle.
In contrast, biological fixation accounts for 90% of the fixed nitrogen in the cycle. In biological fixation, molecular nitrogen (N2) is split into two free N molecules. The N molecules combine with hydrogen (H) molecules to yield ammonia (NH3).
The fixation process is accomplished by a series of different microorganisms. The symbiotic bacteria Rhizobium is associated with the roots of legumes. To a lesser extent, some root-noduled nonleguminous plants also exhibit symbiotic relationships with bacteria. Some free-living aerobic bacteria, such as Azobacter and Clostridium, freely fix nitrogen in the soil. Finally, blue-green algae (cyanobacteria) such as Nostoc and Calothrix can fix nitrogen both in the soil and in water, yielding ammonia as the stable end product.
Ammonification is a one-way reaction in which organisms break down amino acids and produce ammonia (NH3).
Nitrification is the process in which ammonia is oxidized to nitrite and nitrate, yielding energy for decomposer organisms. Two groups of microorganisms are involved in nitrification. Nitrosomonas oxidizes ammonia to nitrite and water. Subsequently, Nitrobacter oxidizes the nitrite ions to nitrate.
Denitrification is the process in which nitrates are reduced to gaseous nitrogen. This process is used by facultative anaerobes. These organisms flourish in an aerobic environment but are also capable of breaking down oxygen-containing compounds (e.g. NO3-) to obtain oxygen in an anoxic environment. Examples include fungi and the bacteria Pseudomonas (Smith, 1990).
In temperate zones, soil nitrate concentrations will vary seasonally with temperature and moisture levels. Fall and winter rains thoroughly remove all nitrates from the soil. No nitrate is naturally added to the soil during the late fall and winter because the cold weather prohibits mineralization and nitrification processes.
During the spring and summer, the increased nitrogen-fixing activity of organisms and the addition of fertilizer causes the concentration of nitrates in the soil to steadily increase. Most of this nitrate is absorbed by plants. Thus, the removal of crops in the fall increases the chance for large flushes of nitrate from the soil to water bodies. Some leaching may occur in the spring if crops are not well- established enough to absorb the nitrogen (Gower, 1980).
The next stage is a direct oxidation step, as follows:
Sixty-six kilocalories of energy are liberated per gram atom of ammonia oxidized.
The biochemical reaction of Nitrobacter is a very simple reaction, involving the cytochrome system as follows:
Then the cyt.Fe+++ is regenerated by:
Eighteen kcal of energy is liberated per gram atom of nitrite oxidized.
This whole process removes electrons from a hydrated nitrite ion. The reactions of Nitrobacter are inhibited by small quantities of ammonia gas (NH3: 1.4 mg/L inhibits 99%), which can lead to a toxic buildup of nitrite, since Nitrosomonas is not
inhibited from oxidizing ammonia to nitrite, in the presence of ammonia.
Both Nitrosomonas and Nitrobacter perform within a pH range of 6.8 to 8.5. Optimal pH is 8.2 to 8.3. Warmer temperatures (above 60°F) also enhance nitrification. The size and type of system, and degree of ammonia present, all influence the prescription dosage and application site. Normally, treatment once or twice weekly with Alken Clear-Flo 1100-50x or Alken Clear-Flo 7110-50x is sufficient, after an adequate biomass is established. Higher initial dosages are usually prescribed to establish a stable biomass rapidly.
Although there are a number
of different strains which will perform nitrification, the rate of formation for Nitrosomonas is typically 1000 to 30,000 mgN/day/g dry weight cells and for Nitrobacter is 5000 to 70000 mgN/day/g dry weight cells, which is so much higher than the formation rates of the other strains capable of nitrification, that these two are the most useful strains. Other bacterial
strains perform nitrification by forming hydroxylamine, amine oxides (R3N-O) or nitroso- compounds (-N-NO or -NOH-NO containing compounds).
The Nitrogen Cycle
The Earth’s atmosphere consists of about 80% nitrogen, in the form of dinitrogen, N2. This molecule is extremely unreactive, containing an N-N triple bond. As such, N2 is unusable as a nitrogen source for all but a few microorganisms, known as nitrogen fixing bacteria. Some of these organisms live in symbiotic relationships with plants: the Rhizobia form such associations with legumes, living in nodules contained on the plants’ roots. These organisms contain the enzyme nitrogenase, responsible for carrying out the reaction which converts N2 to the usable form of ammonia, NH3. The enzyme is imperfect, generating hydrogen gas during the process:
The reaction to produce ammonia from N2 is thermodynamically favorable. The inert nature of N2 is due to a very large activation energy for the reaction to proceed, and therefore requires at least 16 ATP per molecule of N2 which is reduced:
The nitrogenase complex contains an Fe-S reductase, which hydrolyzes two ATP per electron transferred to the Mo-Fe nitrogenase enzyme. The electrons for this reaction come from reduced ferredoxin. Depending on the organism, reduced ferredoxin can be generated by light in photosystem I, or be produced using NADH, H2, or pyruvate. The enzyme is very sensitive to inactivation by oxygen; leguminous plants contain leghemoglobin (a homolog of hemoglobin) in the nodules to bind O2 and maintain a low concentration of it.
Nitrogen typically enters biological systems in the form of ammonia. Most nitrogen in the environment is in the form of N2 gas or as nitrate, NO3-. Plants, fungi and bacteria are able to reduce nitrate to ammonia in a two-step process called nitrate assimilation. This process and nitrogen fixation are the two ways that nitrogen is converted to the usable form of ammonia. Over 99% of the inorganic nitrogen which is assimilated comes from nitrate. Of the remaining 1%, nitrogen fixation accounts for about 60% of the 10 e11 kg/year of nitrogen which enters from N2.
Ammonia can be oxidized by chemoautotrophic bacteria (nitrifying bacteria), using this process as their sole energy source, converting ammonia to nitrate (nitrification). This process and nitrate assimilation go through the intermediate form of nitrite, NO2-. Other anaerobic bacteria can use nitrate as an electron receptor in energy-producing pathways, producing N2 as a final product (denitrification).
These processes are together known as the nitrogen cycle:
The first step of nitrate assimilation is the reduction of nitrate to nitrite by nitrate reductase:
The electrons for this reaction come from NADH and are transferred through enzyme associated sulfhydryl groups, FAD, a cytochrome and a molybdenum cofactor before finally being used to reduce nitrate to nitrite.
The conversion of nitrite to nitrate is carried out by nitrite reductase:
In photosynthetic organisms, the electrons for this reaction come from reduced ferredoxin, passing through an Fe-S protein and a heme group. Microbes use NADPH as an electron donor, passing the electrons through a series of compounds similar to that of nitrate reductase.
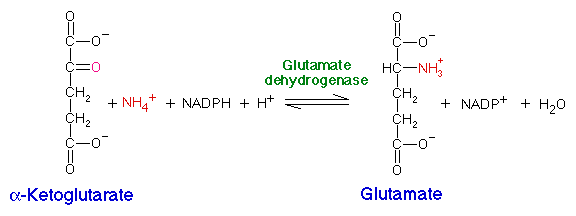
Organisms assimilate nitrogen by incorporating ammonium (NH4+) into amino acids, using glutamate and glutamine as intermediates. Ammonium can be added directly to alpha-ketoglutarate to produce glutamate by glutamate dehydrogenase:
This is the reverse of the deamination reaction in amino acid degradation which uses NAD+.
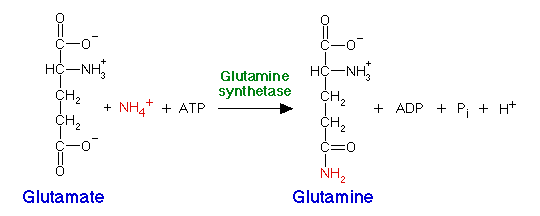
Ammonium can also be incorporated by glutamine synthetase, adding ammonium to glutamate to produce glutamine:
All organisms possess glutamate dehydrogenase and glutamine synthetase. Most bacteria also have glutamate synthase, an enzyme which uses glutamine as the nitrogen donor, converting alpha-ketoglutarate to glutamate:
Glutamine synthetase has a higher affinity for ammonium than glutamate dehydrogenase, and is therefore the enzyme which is used for nitrogen assimilation when ammonium levels are low. In order to regenerate glutamate for further incorporation, glutamate synthase is used. The sum of these reactions is:
Note that this reaction sequence uses both NADPH and ATP to incorporate one molecule of ammonium – the extra energy expenditure is necessary to assimilate nitrogen under limiting conditions. Bacteria which possess both pathways of nitrogen assimilation need to regulate them so that when ammonium levels are high glutamate dehydrogenase is active and glutamine synthetase (GS) and glutamate synthase (GOGAT – glutamate:oxo-glutarate amino-transferase) are not. Conversely, the GS-GOGAT system needs to be activated when ammonium levels are low. This is accomplished through a series of covalent enzyme modifications in response to the relative levels of alpha-ketoglutarate and glutamine. Glutamine synthetase contains 12 identical subunits, each containing a tyrosine residue that can be adenylylated by a regulatory enzyme, referred to as P. P has two forms, PA, which is the form that adenylylates GS, and PD, the form which de-adenylylates GS. PA and PD are interconverted by uridylylation by a uridylyl transferase which is responsive to levels of alpha-ketoglutarate and glutamine.
When ammonium is abundant, levels of glutamine will be high and levels of alpha-ketoglutarate will be low. The uridylyl transferase will remove the uridylyl groups, converting PD to PA. PA will then adenylylate glutamine synthetase, inactivating it.
When ammonium levels are low, levels of alpha-ketoglutarate will be high and levels of glutamine will be low. The uridylyl transferase uridylylates PA, converting it to PD under these conditions. PD activity leads to the phosphorolysis of the adenylylated form of glutamine synthetase, which activates it.
The adenylyated form of glutamine synthetase is inactive because it is much more sensitive to cumulative feedback inhibition by the products of glutamine metabolism. The amide group of glutamine is used as a nitrogen source in the biosynthesis of tryptophan, histidine, carbamoyl phosphate, glucosamine 6-phosphate, CTP, and AMP. All of these products as well as alanine and glycine can act to inhibit the enzyme.
The cascade of regulatory enzymes acts to amplify the signal as well as to allow more sites for allosteric control because each enzyme can be independently regulated.
In addition to covalent modification and feedback inhibition, glutamine synthetase is transcriptionally regulated by an enhancer which is active when in a phosphorylated form. The enzyme which phosphorylates this enhancer acts as a phosphatase when complexed with PA, which is present when glutamine synthetase activity is not needed. The net result is reduced transcription of glutamine synthetase when ammonium levels are adequate, and increased transcription when ammonium levels are low.